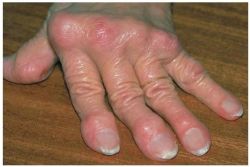
An FDA advisory panel has recommended the approval of a new drug to treat people with rheumatoid arthritis.
The drug is called Actemra, and is manufactured by Swiss drug maker Roche.
It works by blocking a protein called interleukin-6 which plays a role in inflammation in the body.
The FDA does not have to side with their advisory panels when it comes to approving or not approving drugs, but they usually do side with them.
There are some safety concerns with the drug, but the panel believes that the risks far are outweighed by the benefits.
“The safety concerns are real, and I think there is going to have to be monitoring,” said panel member Dr. David Felson, clinical epidemiology chief at Boston University School of Medicine.
Leave a Reply